On April 1, 2024, Prof. Bin Zhao’s laboratory published a paper entitled Nuclear KRT19 is a transcriptional co-repressor promoting histone deacetylation and liver tumorigenesis in Hepatology. This study uncovered a novel mechanism whereby Keratin 19 (KRT19), thought to be merely a structural protein forming the keratin cytoskeleton, has a previously unrecognized nuclear function in regulating gene expression as a cancer-specific component of the CoREST complex.
Primary liver cancer is the fourth leading cause of cancer death worldwide, and treatment options are very limited. Hepatocellular carcinoma (HCC) is the most common type of liver cancer. Dedifferentiation is a frequent event during the development of HCC. Some HCC cells will exhibit characters of liver cancer stem cells, and express marker genes such as KRT19. Despite the recognition of an association between KRT19 expression and worse prognosis, its functional roles in HCC in vivo have not been demonstrated.
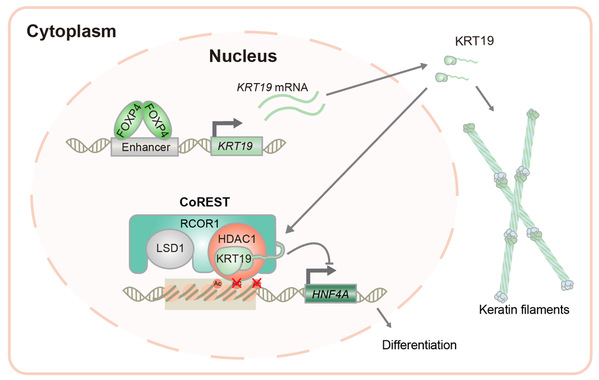
Using multiplexed genome editing of hepatocytes in vivo, Prof. Zhao’s group demonstrated that KRT19 promoted liver tumorigenesis in mice. Cell fractionation revealed a previously unrecognized nuclear fraction of KRT19. Tandem affinity purification identified histone deacetylase 1 (HDAC1) and REST co-repressor 1 (RCOR1), components of the co-repressor of RE-1 silencing transcription factor (CoREST) complex as KRT19-interacting proteins. KRT19 knockout markedly enhanced histone acetylation levels. Mechanistically, KRT19 promotes CoREST complex formation by enhancing HDAC1 and RCOR1 interaction, thus increases the deacetylase activity. ChIP-seq revealed hepatocyte-specific genes, such as hepatocyte nuclear factor 4 alpha (HNF4A), as direct targets of KRT19-CoREST. In addition, they identified forkhead box P4 (FOXP4) as a direct activator of aberrant KRT19 expression in liver cancer. Furthermore, treatment of primary liver tumors and patient-derived xenografts in mice suggest that KRT19 expression has the potential to predict response to HDAC inhibitors especially in combination with Lenvatinib.
This study revealed that nuclear KRT19 inhibits the expression of hepatocyte specific genes as a transcription co-repressor by promoting the deacetylase activity of the CoREST complex, leading to HCC dedifferentiation. These findings reveal the function of KRT19 in HCC epigenetic reprogramming. Dr. Shixun Han, Haonan Fan, and Guoxuan Zhong are co-first authors, and the corresponding author is Prof. Bin Zhao. This work is a collaboration with Profs. Dong Fang and Xin-Hua Feng from LSI, Zhejiang University, Chen Ding and Yanhui Xu from Fudan University, and Tingbo Liang from the First Affiliated Hospital of Zhejiang University. This work was supported by grants to Prof. Bin Zhao from the National Natural Science Foundation of China, Natural Science Foundation of Zhejiang, and the Fundamental Research Funds for the Central Universities.
Article link:
https://journals.lww.com/hep/abstract/9900/nuclear_krt19_is_a_transcriptional_co_repressor.832.aspx



