Li Shen’s laboratory recently published a research article entitled “Genomewide decoupling of H2AK119ub1 and H3K27me3 in early mouse development” in Science Bulletin as the cover story, reporting an unexpected decoupling phenomenon of the two closely connected histone marks in early embryos.

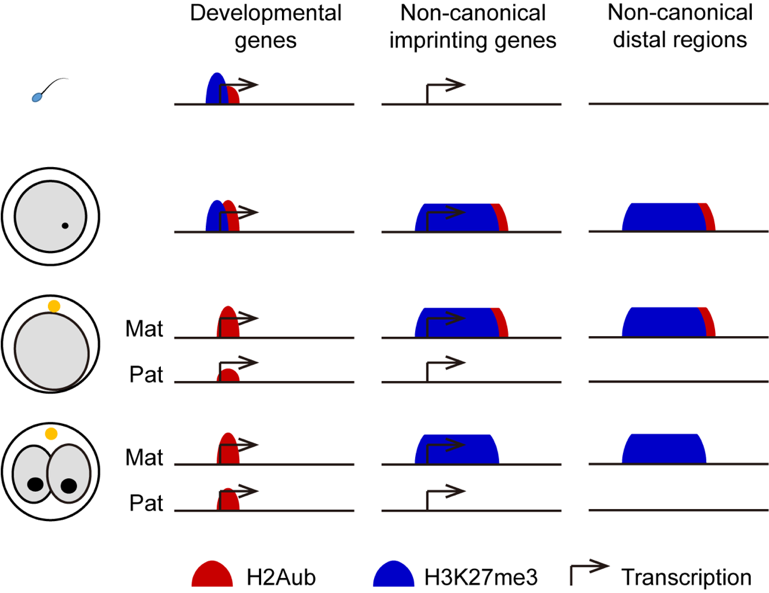
Polycomb group (PcG) is a family of important protein complexes first discovered in Drosophila that can play a role in chromatin remodeling and epigenetic silencing of genes. Mono-ubiquitination of histone H2A at lysine 119 (H2AK119ub1 or H2Aub) and tri-methylation of histone H3 at lysine 27 (H3K27me3) are deposited by Polycomb-repressive complex 1 (PRC1) and PRC2, respectively. The two intimately associated histone modifications play central roles in Polycomb group protein (PcG)-mediated transcriptional repression and are of paramount importance to mammalian development. While a classic model suggested recruitment of PRC1 by PRC2-mediated H3K27me3 deposition, recent studies have also demonstrated the recruitment of PRC2 by PRC1-mediated H2Aub deposition, suggesting a reciprocal recognition of H2Aub and H3K27me3 by PRC2 and PRC1 respectively. Indeed, PRC1/H2Aub and PRC2/ H3K27me3 have been reported to largely overlap in the genome, particularly at canonical PcG targets (i.e., the promoters of bivalent genes, mainly developmental related genes). However, whether they repress PcG target genes cooperatively or redundantly is still under debate, and it is largely unclear whether they also function independently at different genomic regions in some biological contexts.
In addition to decorating canonical PcG targets, H3K27me3 has also been reported to form special pattern at broad distal domains in oocytes, which are inherited by early embryos to mediate DNA methylation-independent non-canonical imprinting. Remarkably, the ectopic removal of H3K27me3 resulted in the loss of non-canonical imprinting, suggesting that H3K27me3 plays a dominant role in silencing the maternal allele of non-canonical imprinted genes. However, due to the interdependent recruitment of PRC1 and PRC2, it is unknown whether H2Aub also contributes to this DNA methylation-independent non-canonical imprinting system.
Whether H2Aub and H3K27me3 could function independently in different genomic regions and whether H2Aub could play a role in DNA methylation-independent non-canonical imprinting are still unclear, as the genome-wide enrichment of H2Aub in mouse early embryos has not been addressed due to the limitation of sequencing methods at low number of embryos. This study developed an ultra-sensitive chromatin immunoprecipitation sequencing (ChIP-Seq) method termed CATCH-Seq (carrierDNA-assisted ChIP-Seq) and generated allelic H2Aub profiles in mouse gametes and early embryos. The data shows that H2Aub exhibited similar genomic distribution to H3K27me3 in mouse oocytes and sperm, but distinct distribution after fertilization, suggesting an unexpected genomewide decoupling of H2Aub and H3K27me3 in mouse preimplantation embryos, where H2Aub but not H3K27me3 was enriched at PcG targets while only H3K27me3 was deposited in the broad distal domains associated with DNA methylation-independent non-canonical imprinting. These observations suggest that H2Aub represses future bivalent genes during early embryogenesis without H3K27me3, but it is not required for the maintenance of non-canonical imprinting, which is mediated by maternal H3K27me3.
This work was supported by the National Natural Science Foundation of China, the National Key Research and Development Programs of China, and Zhejiang Provincial Natural Science Foundation of China. Yezhang Zhu and Jiali Yu from the Life Sciences Institute at Zhejiang University are the co-first authors of this article.
Link:https://www.sciencedirect.com/science/article/pii/S2095927321004151



