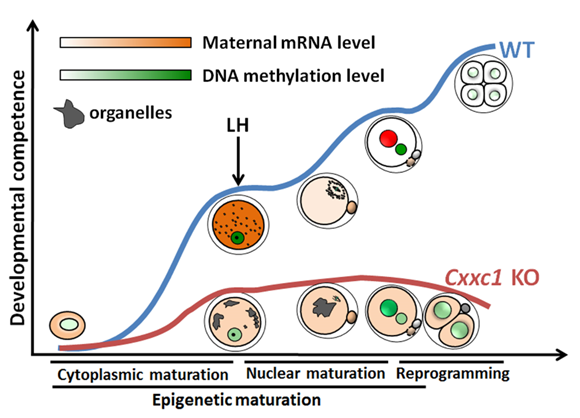
Trimethylation of histone H3 at lysine-4 (H3K4me3) is associated with eukaryotic gene promoters and poises them for development- or environment-triggered transcriptional activation. To address the in vivo function of H3K4me3 in the absence of DNA replication, professor Heng-Yu Fan’s group deleted CXXC finger protein1 (CFP1), the DNA-binding subunit of SETD1 histone H3K4 methyltransferase, in developing oocytes. Their results showed that CFP1 is required for H3K4me3 accumulation and the deposition of histone variants onto chromatin during oocyte maturation. A decrease of H3K4me3 in oocytes caused global down regulation of genome transcription activity. Oocytes lacking CFP1 failed to complete epigenetic maturation and were unable to gain developmental competence after fertilization, because of the defects in cytoplasmic lattice formation, meiotic division and maternal-zygotic transition. This study highlights the importance of H3K4me3 in continuous histone replacement for transcriptional regulation, chromatin remodeling, and normal developmental progression in a non-replicative system.
Link: http://www.cell.com/cell-reports/fulltext/S2211-1247(17)30964-6



