The research group led by Prof. Xin-Hua Feng from Life Sciences Institute (LSI), Zhejiang University has recently published a paper entitled “Tumor suppressor bromodomain-containing protein 7 cooperates with Smads to promote transforming growth factor-β responses” in Oncogene on June 6, 2016.
Transforming growth factor-β(TGF-β) is a ubiquitously expressed cytokine that controls a plethora of cellular responses from cell proliferation, differentiation and apoptosis to embryonic development in metazoan.Smad proteins are central mediators in the canonical TGF-β signaling pathway in mammalian cells. Feng lab found that bromodomain-containing protein 7 (BRD7) forms a TGF-β inducible complex with Smad3/4 through its N-terminal Smad-binding domain and functions as a novel transcription coactivator for Smads in TGF-β signaling. Mechanically, BRD7 simultaneously binds to acetylated histones to promote Smad-chromatin association, and associates with histone acetyltransferase p300 to enhance Smad transcriptional activity.
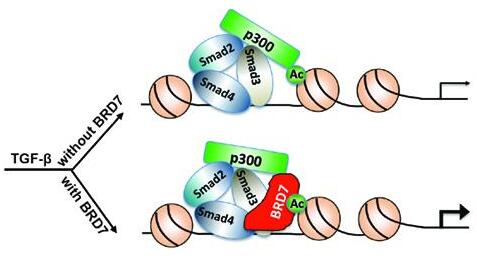 |
| Figure: A working model for the function of BRD7 in TGF-β signaling. BRD7 binds to acetylated chromatin upon TGF-β treatment to promote Smad4-DNA binding ability and cooperates with p300 to enhance Smad transcriptional activity. |
BRD7 has been reported to inhibit cell proliferation and promote cell cycle arrest through the p53 and the PI3K pathways, yet there are conflicting results about the absolute role of these pathways in mediating physiological functions of BRD7. The data provided by Dr. Feng’s lab suggested a new function to BRD7 in cell growth regulation through the TGF-β pathway. Given the involvement of BRD7 in multiple pathways, BRD7 may converge these signaling pathways to function in cell growth control, development and tumorigenesis.
Text link:http://www.nature.com/onc/journal/vaop/ncurrent/full/onc2016204a.html



